Human beta-amyloid (1-42) protein, also known as Aβ 1-42, is a key factor in unlocking the mysteries of Alzheimer’s disease. This peptide plays a central role in the formation of amyloid plaques, enigmatic clusters that damage the brains of Alzheimer’s patients. With a destructive effect, it disrupts neuronal communication, triggers inflammation, and induces neurotoxicity, leading to cognitive impairment and neural damage. Investigating its aggregation and toxicity mechanisms is not only vital; it’s an exciting journey toward solving Alzheimer’s puzzle and developing future therapies.



Aβ 1-42 is a peptide fragment of 42 amino acids that is derived from the cleavage of the amyloid precursor protein (APP) by β- and γ-secretases. Aβ 1-42 is one of the main components of the amyloid plaques that accumulate in the brains of patients with Alzheimer’s disease, a neurodegenerative disorder characterized by cognitive impairment and memory loss. Aβ 1-42 has been shown to have various functions and applications in biological and biomedical research, such as:
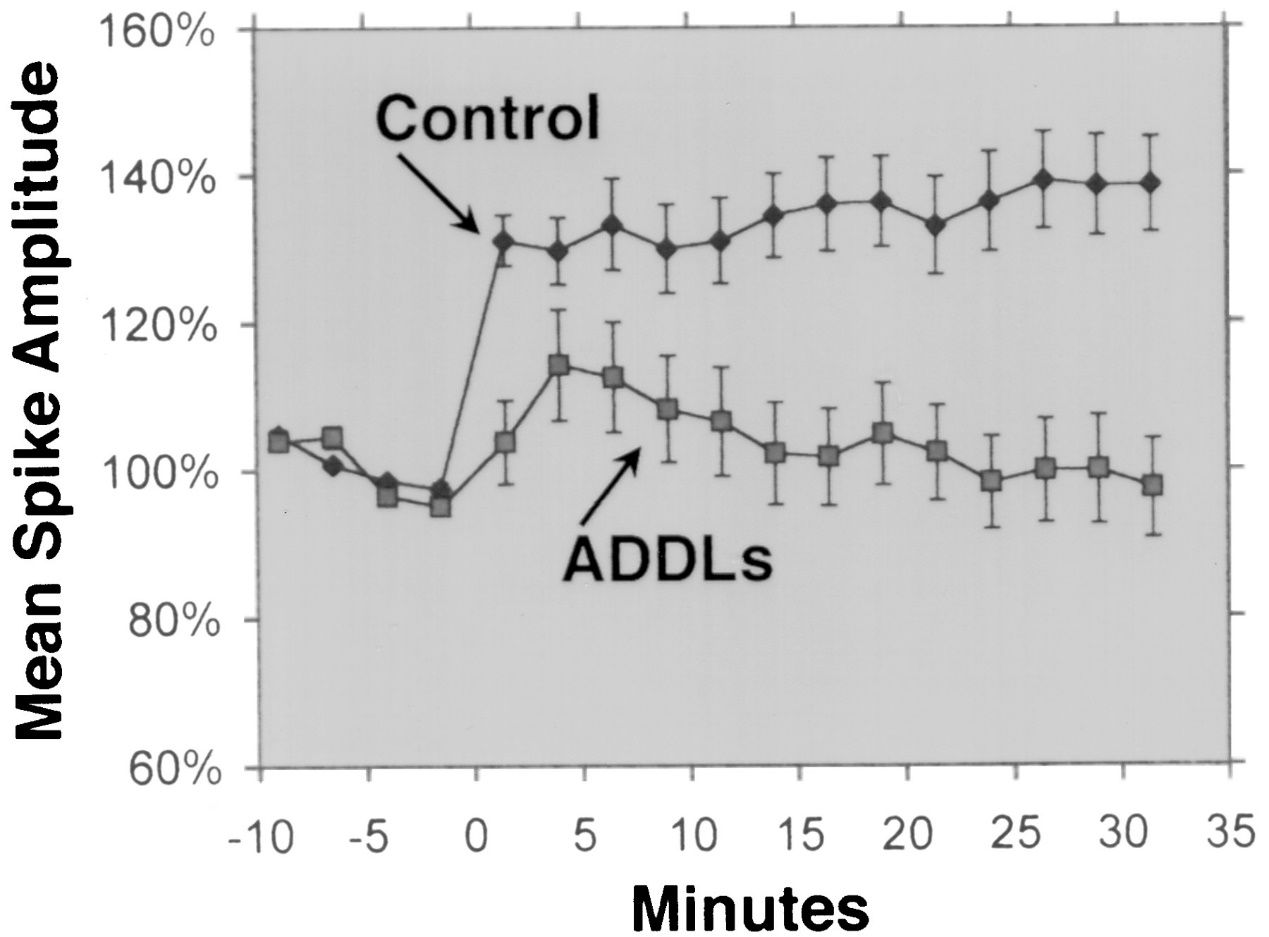
1.Neurotoxicity: Aβ 1-42 can form soluble oligomers that are capable of binding to and disrupting the function of neuronal membranes, receptors, and synapses. These oligomers can also induce oxidative stress, inflammation, and apoptosis in neurons, leading to synaptic loss and neuronal death. Aβ 1-42 oligomers are considered to be more neurotoxic than other forms of Aβ, such as Aβ 1-40, which is the most abundant form of Aβ in the brain. Aβ 1-42 oligomers are also thought to be able to propagate from cell to cell, similar to prions, and trigger the misfolding and aggregation of other proteins, such as tau, which forms neurofibrillary tangles in Alzheimer’s disease.
Aβ 1-42 is widely regarded as the Aβ isoform with the highest neurotoxicity. Several experimental studies have demonstrated the neurotoxicity of Aβ 1-42 using different methods and models. For example, Lesné et al. (Brain, 2013) investigated the formation and toxicity of Aβ oligomers, which are soluble aggregates of Aβ monomers, and showed that Aβ 1-42 oligomers had a stronger damaging effect on neuronal synapses, leading to cognitive decline and neuronal loss. Lambert et al. (Proceedings of the National Academy of Sciences, 1998) highlighted the neurotoxicity of Aβ 1-42 oligomers and found that they had a strong toxic effect on the central nervous system, possibly by affecting synapses and neurotransmitters. Walsh et al. (Nature, 2002) showed the inhibitory effect of Aβ 1-42 oligomers on hippocampal long-term potentiation (LTP) in vivo, which is a cellular mechanism underlying learning and memory. This inhibition was associated with memory and learning impairment, emphasizing the impact of Aβ 1-42 oligomers on synaptic plasticity. Shankar et al. (Nature Medicine, 2008) isolated Aβ 1-42 dimers directly from Alzheimer’s brains and showed their effect on synaptic plasticity and memory, providing empirical evidence for the neurotoxicity of Aβ 1-42 oligomers.
In addition, Su et al. (Molecular & Cellular Toxicology, 2019) performed transcriptomics and proteomics analysis of Aβ 1-42-induced neurotoxicity in SH-SY5Y neuroblastoma cells. They identified several genes and proteins that were affected by Aβ 1-42 in pathways related to apoptotic process, protein translation, cAMP catabolic process and response to endoplasmic reticulum stress. Takeda et al. (Biological Trace Element Research, 2020) investigated the role of extracellular Zn2+ in Aβ 1-42-induced neurotoxicity in Alzheimer’s disease. They showed that Aβ 1-42-induced intracellular Zn2+ toxicity was accelerated with aging because of age-related increase in extracellular Zn2+. They suggested that Aβ 1-42 secreted continuously from neuron terminals causes age-related cognitive decline and neurodegeneration via intracellular Zn2+ dysregulation. These studies suggest that Aβ 1-42 is a key factor in mediating neurotoxicity and disease progression in Alzheimer’s disease by affecting various molecular and cellular processes in the brain.
2. Antimicrobial activity: Aβ 1-42 has been reported to have antimicrobial activity against various pathogens, such as bacteria, fungi, and viruses. Aβ 1-42 can bind to and disrupt the membranes of microbial cells, leading to their lysis and death. Aβ 1-42 can also activate the innate immune system and recruit inflammatory cells to the site of infection. Some studies have suggested that the accumulation of Aβ in the brain may be a protective response to chronic infections or injuries. However, excessive or dysregulated production of Aβ may also cause collateral damage to the host cells and tissues.
Aβ 1-42 has been reported to exhibit antimicrobial activity against a range of pathogens, such as bacteria, fungi, and viruses, such as Staphylococcus aureus, Escherichia coli, Candida albicans, and Herpes simplex virus type 1, by interacting with their membranes and causing their disruption and lysis. Kumar et al. (Journal of Alzheimer’s Disease, 2016) demonstrated this effect by showing that Aβ 1-42 altered the membrane permeability and morphology of microbial cells, leading to their death. In addition to its direct antimicrobial action, Aβ 1-42 can also modulate the innate immune response and recruit inflammatory cells to the site of infection. Soscia et al. (PLoS One, 2010) revealed this role by reporting that Aβ 1-42 stimulated the production of pro-inflammatory cytokines and chemokines, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-1 alpha (MIP-1α), in microglia and astrocytes, the main immune cells in the brain.
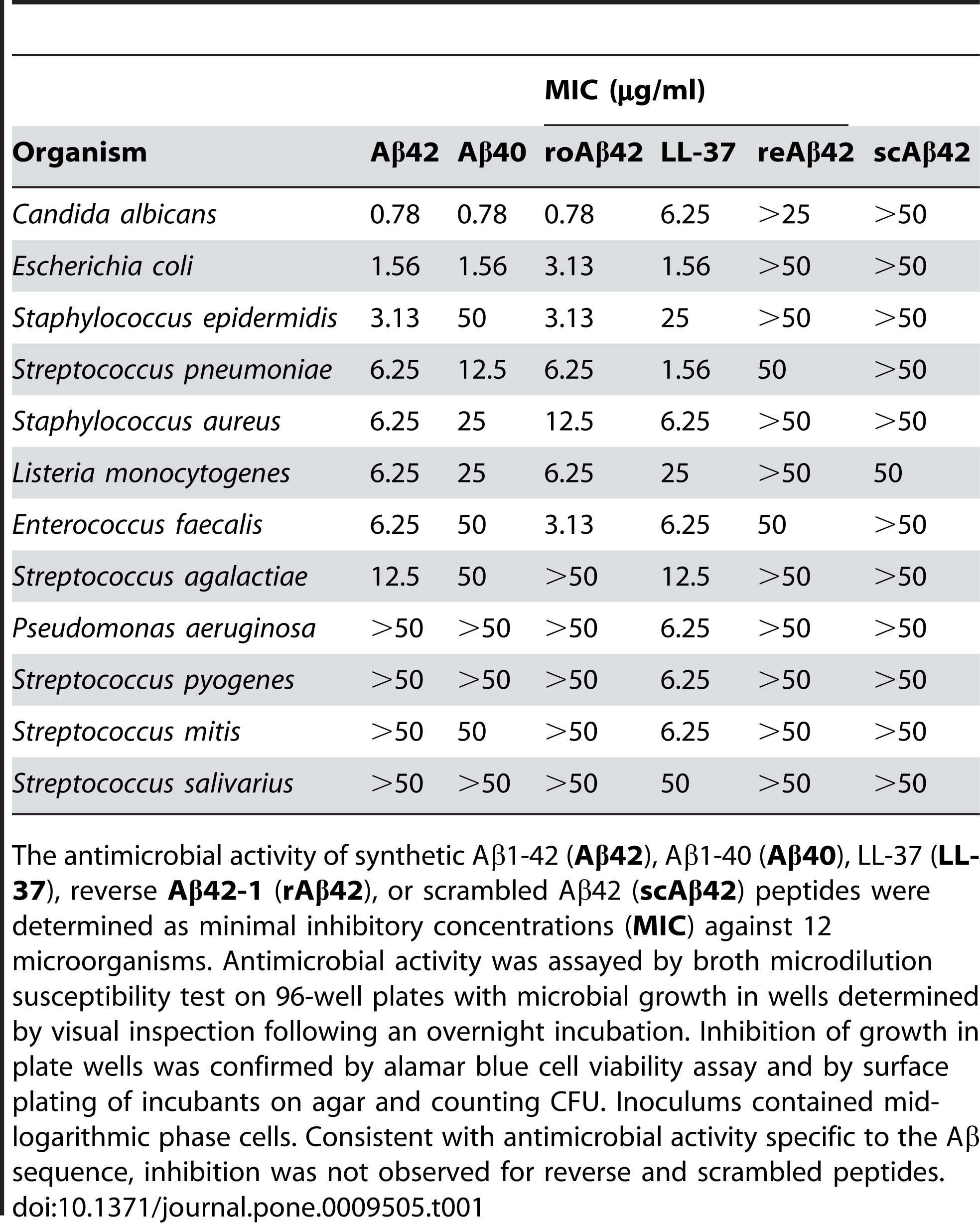
Figure 2. Aβ peptides possess antimicrobial activity.
(Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010 Mar 3;5(3):e9505.)
While some studies have suggested that the accumulation of Aβ in the brain may be a protective response to chronic infections or injuries, as Aβ can act as an antimicrobial peptide (AMP) and eliminate potential pathogens, the complex interplay between Aβ and microbial elements remains a topic of investigation. The delicate balance is highlighted by the research of Moir et al. (Journal of Alzheimer’s Disease, 2018), which suggests that imbalanced or excessive Aβ production may inadvertently harm host cells and tissues, reflecting the intricate dual nature of Aβ’s roles in infection and neurodegeneration. Excessive or dysregulated production of Aβ may lead to its aggregation and deposition in the brain, forming toxic oligomers and fibrils that impair neuronal function and induce neuroinflammation. These pathological processes are associated with cognitive decline and memory loss in Alzheimer’s disease, a neurodegenerative disorder characterized by progressive dementia. Therefore, the balance between the beneficial and detrimental effects of Aβ is crucial for maintaining brain health and preventing neurodegeneration.
3.Iron export: Aβ 1-42 has been shown to be involved in the regulation of iron homeostasis in the brain. Iron is an essential element for many biological processes, but excess iron can also cause oxidative stress and neurodegeneration. Aβ 1-42 can bind to iron and facilitate its export from neurons via ferroportin, a transmembrane iron transporter. This may help prevent iron accumulation and toxicity in the brain, as excess iron can cause oxidative stress and neurodegeneration. Duce et al. (Cell, 2010) reported that Aβ 1-42 bound to ferroportin and increased its expression and activity in neurons, leading to reduced intracellular iron levels. They also showed that Aβ 1-42 reduced the expression of hepcidin, a hormone that inhibits ferroportin, in astrocytes, further enhancing iron export from neurons. However, iron-bound Aβ may also become more prone to aggregation and deposition in the extracellular space, forming amyloid plaques. Ayton et al. (Journal of Biological Chemistry, 2015) reported that iron promoted the formation of Aβ oligomers and fibrils in vitro and in vivo. They also showed that iron chelation reduced Aβ aggregation and deposition in transgenic mice. Therefore, the balance between the beneficial and detrimental effects of Aβ 1-42 on iron homeostasis is crucial for maintaining brain health and preventing neurodegeneration.
We are a polypeptide manufacturer in China, with several years of mature experience in polypeptide production. Hangzhou Taijia Biotech Co., Ltd. is a professional polypeptide raw material manufacturer, which can provide tens of thousands of polypeptide raw materials and can also be customized according to needs. The quality of polypeptide products is excellent, and the purity can reach 98%, which has been recognized by users all over the world.Welcome to consult us.






